Single crystals at high temperatures can radically change the lifespan of electric vehicles
A research team led by Professor Kyu-Young Park from the Graduate Institute of Ferrous and Eco Materials Technology and the Department of Materials Science and Engineering, alongside Kyoung Eun Lee, a PhD candidate, and alumna Yura Kim from the Graduate Institute of Ferrous and Eco Materials Technology at Pohang University of Science and Technology (POSTECH) has made significant progress in the synthesis of monocrystalline cathode materials for electric vehicles. This breakthrough, achieved in collaboration with the POSCO Holdings N.EX.T Hub, was published in ACS Materials and Interfaces.
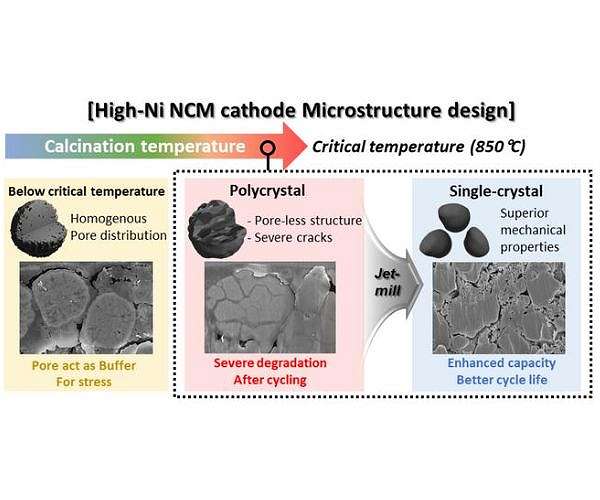
Lithium (Li) secondary batteries, common in electric vehicles, function by converting electrical energy into chemical energy and vice versa through the movement of Li ions between the cathode and anode. Nickel (Ni) cathode materials are often used because of their high storage capacity for lithium ions. However, traditional nickel-based materials have a polycrystalline structure consisting of numerous small crystals, which can be broken down during charging and discharging, shortening their lifespan.
To overcome this limitation, the researchers developed a method to produce nickel-based cathode materials in a “single-crystal” form. These single crystals are synthesized as large particles, improving their structural and chemical stability and durability. Although single-crystal materials are known to be hardened at high temperatures, the specifics of this process and the conditions required were previously unclear.
The research team wanted to identify the “critical temperature” needed to synthesize high-quality monocrystalline materials. They experimented with different temperatures to optimize synthesis conditions for nickel-based cathode material (N884), observing the effects on long-term capacity and performance.
They found that polycrystalline materials synthesized below a certain temperature degrade more quickly in secondary batteries. However, when synthesized above this critical temperature, high-quality single crystals are formed, greatly improving durability. This improvement is attributed to a process called ‘densification’, in which the internal grain size increases and voids in the material are densely filled, resulting in extremely hard and degradation-resistant single crystals.
Professor Kyu-Young Park explained: “We have introduced a new synthesis strategy to improve the durability of nickel-based cathode materials.” He added: “We will continue our research to make secondary batteries for electric vehicles cheaper, faster and more sustainable.”
The study received support from POSCO Holdings and the Basic Research Program of the Ministry of Science and ICT.