Battery diagram
Image: Pohang University of Science and Technology (POSTECH), Nature Communication, Common License CC BY 4.0
From pv magazine ESS news place
Metal-air batteries, using lithium or sodium, have been of great interest because of their exceptionally high theoretical gravimetric energy densities. These batteries rely primarily on the use of pure oxygen for the formation and breakdown of metal oxides, rather than ambient air.
Although ambient air is a more practical option, CO2 and H2O present in the air causes serious irreversible reactions, such as the formation of carbonates and hydroxides, which usually damage a battery. To address this, metal-air batteries usually require additional equipment such as an oxygen permeation membrane to purify oxygen or selectively use oxygen from the air.
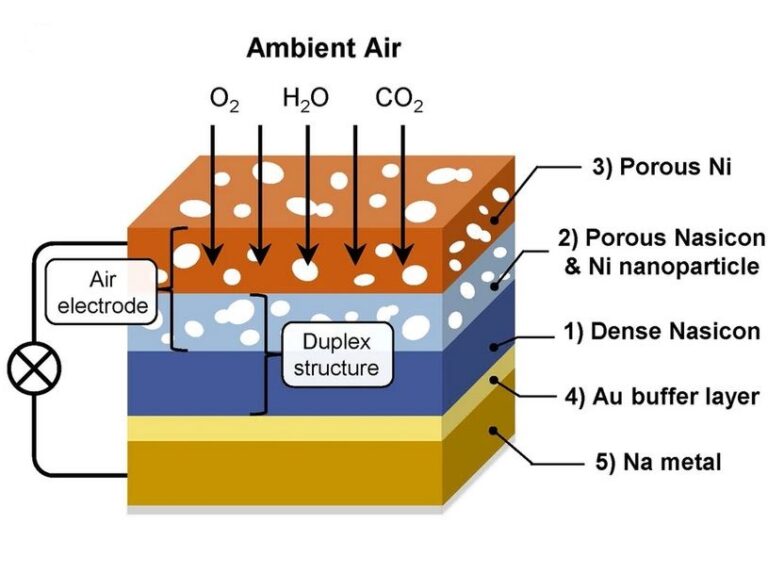
Now, researchers at Pohang University of Science and Technology (POSTECH) in South Korea have developed a high-energy, high-efficiency, all-solid sodium-air battery that can reversibly use sodium and air without the need for special equipment.
The team used Nasicon, a superionic sodium conductor and a solid electrolyte, to tackle the carbonate problem. Nasicon, which contains elements such as sodium, silicon and zirconium, enables ion movement in the solid state while exhibiting high electrochemical and chemical stability.
By using this solid electrolyte, the team protected sodium metal electrodes from air and facilitated the breakdown of carbonate formed during the operation of the electrochemical cell.
To read further, visit our ESS news website.
This content is copyrighted and may not be reused. If you would like to collaborate with us and reuse some of our content, please contact: editors@pv-magazine.com.