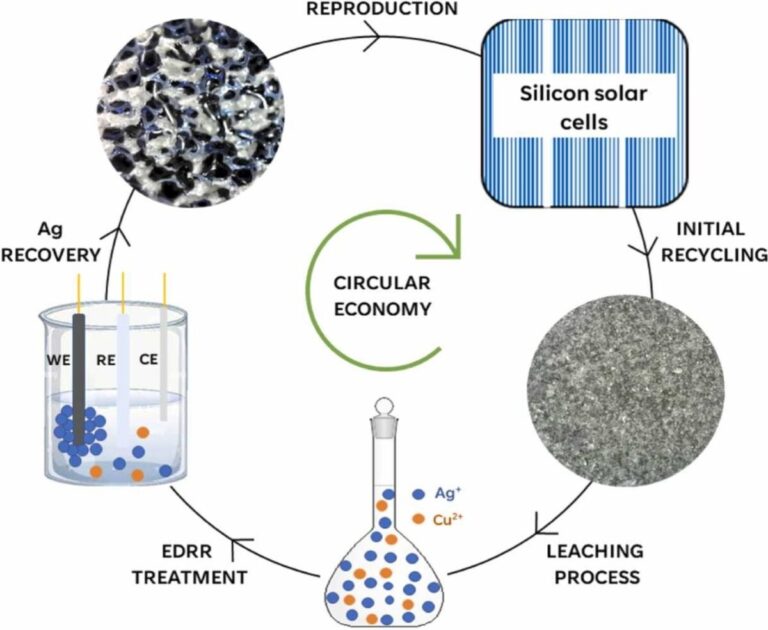
Scientists have used hydrometallurgical and electrochemical processes to extract pure silver from solar cells. The proposed technique also uses a method known as electrodeposition redox replacement, which reportedly increases the silver recovery rate.
Researchers from Italy’s University of Camerino have developed a new way to recover silver from discarded solar cells.
By combining hydrometallurgical and electrochemical processes, they were reportedly able to recover pure silver with an efficiency of 98%. Hydrometallurgical processes, or leaching, use aqueous solutions to extract metals, while electrochemical processes refer to the use of electric currents to drive reactions in metals.
“Recovery of raw materials is crucial for several reasons,” the academics explained. “Conventional metal extraction techniques, such as open-pit mining, can cause significant damage to the environment and adjacent ecosystems. Therefore, using a metal recovery process based on industrial waste can mitigate the environmental impacts associated with metal production. In addition, recovering metals generally requires less energy than extracting metals from ore, leading to lower energy consumption and lower CO2 emissions, especially if the recovery rate is comparable to or higher than traditional extraction methods.”
Due to the narrow values of the standard reduction potential of silver and copper, leaching silver particles from PV waste is challenging. To overcome this, the researchers proposed a combined base-activated persulfate and ammonia, where persulfate acts as an oxidant, while the system itself generates a protective hermetic layer of copper(II) oxide, preventing its own leaching.
To test the proposed process, the researchers conducted an experiment consisting of several parameters for the leaching process. These were the concentration of ammonia (NH₃) in a solution, measured in moles per liter (mol/L); PV waste sample, in grams per liter (g/l); potassium persulphate (PPS), in mol/l; and response time, in minutes. The temperature was maintained at 25°C and the stirring speed at 300 rpm during all experiments.
Image: University of Camerino,
Environmental technology and innovation, CC BY 4.0
“The following conditions were chosen as best: 0.5 M NH₃, 0.2 mol/L PPS, S/L ratio up to 50 g/L and reaction time set to 60 minutes,” the academics explained. “Two additional experiments were performed under these conditions to assess reproducibility. In combination with the two previous tests from the experimental design, a mean of 85.0 ± 2.6% was achieved at a 95% confidence interval.
Despite these good results, 85% of the recovered pure silver was considered insufficient by the research group, which decided to initiate the electrochemical process. Namely, the electrodeposition redox replacement (EDRR) approach using a pulsed electrodeposition method. “This technique, previously described in the literature, allows the recovery of highly pure silver metal from hydrometallurgical leachate containing copper ions,” the team explains.
The best conditions for silver recovery were found to be the EDRR approach, which reportedly achieved an efficiency of 98.7%. “It is noteworthy to mention that this approach appears to be selective in silver recovery and does not require any chemical additions. This unique feature makes this methodology competitive compared to conventional processes,” the scientists concluded.
The new method and the results of the experiments were presented in “Silver recovery from silicon solar cell waste using hydrometallurgical and electrochemical techniques”, published in Environmental technology and innovation. The research was carried out in collaboration with ORIM, an Italian company specialized in recovering metals from solid waste.
This content is copyrighted and may not be reused. If you would like to collaborate with us and reuse some of our content, please contact: editors@pv-magazine.com.
Popular content